Professor Sir Stephen O'Rahilly, Director
The MRC Metabolic Diseases Unit (MDU) was established in 2013. Recognising that obesity and its adverse health consequences were a major and growing threat to public health globally, the MRC identified the strategic benefits of co-localising a new Unit, focused on studying the causes and consequence of obesity at a biologically mechanistic level, with its existing investment in the population science and public health aspects of the disorders, namely the MRC Epidemiology Unit.
Partnering with the Wellcome Trust, a joint Institute of Metabolic Science was established. This has powerfully fostered cross interactions with many projects linking work in molecules, animal models and deeply phenotyped human participants, with large-scale population-level analyses. This multidisciplinary approach continues to deliver many important and novel scientific insights.
The MRC MDU directly supports five research programmes:
- Mechanisms in disorders of energy balance and body composition (Dr Anthony Coll, Professor Sir Stephen O’Rahilly and Professor Giles Yeo)
- Adipose tissue dysfunction and lipotoxicity: Uncoupling obesity from associated metabolic comorbidities (Professor Antonio Vidal Puig)
- Neurophysiology of gut hormones (Professor Fiona Gribble and Professor Frank Reimann)
- Feto-maternal communication and the developmental programming of metabolic disease (Professor Susan Ozanne and Dr Miguel Constancia)
- Nutrient sensing in the brain (Dr Clemence Blouet)
The MDU, together with funding from Wellcome and NIHR BRC also supports key core facilities within the IMS that underpin much of the science of the Unit and Institute.
Programme leaders - Dr Anthony Coll, Professor Sir Stephen O’Rahilly and Professor Giles Yeo
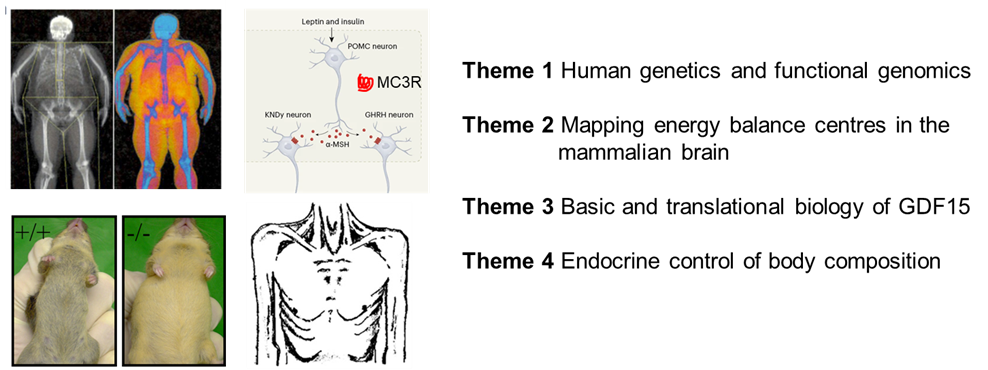
Disorders of body weight and body composition remain a major public health issue with a continued need for improved therapy and prevention. Obese people suffer increase mortality and morbidity from a range of cardiovascular and metabolic disorders while chronic disease cachexia, characterised by progressive loss of lean and fat mass, is associated with reduced survival and reduced tolerance to therapy. Work on this programme will cover four distinct themes which together will increase mechanistic understanding of the drivers behind disorders of appetitive behaviour and body composition relevant to human disease.
Specifically, in Theme 1 (human genetics and functional genomics studies) we will use insights from human and few genetics to define molecular mechanisms in human obesity. We will continue fruitful collaborations with cohorts at extremes of adiposity, including those enriched with consanguineous pedigrees, and with the Genes and Health cohort, the world's largest community-based genetic studies. We will build upon our recent discovery that rare loss of function mutations in the scaffold protein BSN are linked to body weight through functional studies in neuronal and intestinal cells. Theme 2 will continue to map energy balance centres in the mammalian brain, building on our expertise with a view to map cellular architecture and spatial gene expression in the obese human hindbrain, with a particular focus on nuclei (NTS and AP) implicated in appetite control. We will continue to work intensively on GDF15 in Theme 3, focusing on mechanism underlying the desensitisation of behavioural response to GDF15 by prior exposure defined by our previous work on the nausea and vomiting of pregnancy. In particular, we will undertake cellular and whole animal studies to more fully define the central site of this phenomenon as well as and defining the wider impact of GDF15 on neuroendocrine axes. Finally, in Theme 4 we will examine the role of Activin A, a member of the TGFb, as a potential neuroendocrine modulator of skeletal muscle metabolism. Although recognised to be elevated in human cancer cachexia syndrome, its physiological role remains elusive. We hypothesise Activin A is an endocrine product of the liver which acts to modulate skeletal muscle metabolism in response to hepatic nutritional stress and will look to define this further using a series of model organism and cellular systems.
Lay summary:
A wide range of different human diseases are driven by problems of body composition. Both the amount of fat and the body site of the fat store profoundly influence the chances of developing diseases such as diabetes. Further, conditions where muscle mass is poorly maintained can also lead to significant medical problems.
Our research focuses on working out what determines how much we eat and what we do with those nutrients once they are in our body. We know from our previous work that the brain is a key controller in all of these processes and relies greatly on receiving hormone ”messenger signals” from other parts of the body to do that job, but we want to find out more so we can design better therapies. We will study in detail humans who have unusually high levels of body fat stores and combined that with modern, powerful laboratory techniques to understand their genetic makeup and how their genes may be contributing to their body weight. We will combine this work by looking to map out the architecture of these controlling centres in the human brain.
Using the information from these human studies, we will turn to laboratory studies using cells and in vivo model systems to better understand some of the most promising messenger molecules that have the potential to meaningfully alter appetite, body fat and body lean mass in human disease.
Programme leader: Professor Antonio Vidal Puig
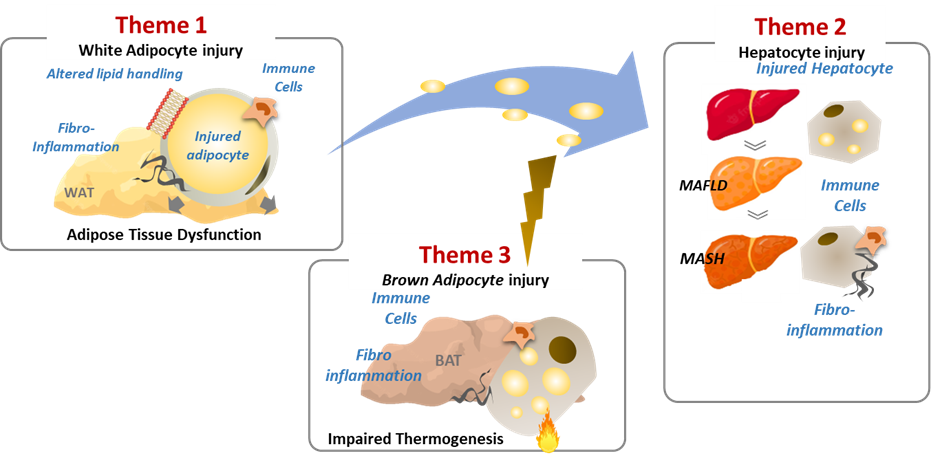
This programme addresses the complex relationship between obesity and metabolic comorbidities through innovative research and therapeutic strategies. It introduces key concepts such as the "adipose tissue expandability model" and "lipotoxicity" to deepen our understanding of how obesity contributes to metabolic complications. The research focuses on the mechanisms where injured or dysfunctional white adipocytes in obese individuals trigger immune-mediated fibroinflammatory responses in metabolic organs like adipose tissue, liver, and brown fat. The aim is to uncover mechanisms that decouple excessive fat mass accumulation from ectopic lipid accumulation and systemic inflammation associated with obesity. The programme is structured into three themes:
- Mechanisms of Adipose Tissue Dysfunction and Fibro-Inflammation: Investigating molecular mechanisms behind adipocyte dysfunction and injury leading to adipose tissue inflammation.
- Pathways Linking Adipose Tissue Dysfunction to Systemic Comorbidities: Focusing on mechanisms linking adipose tissue dysfunction to conditions like non-alcoholic fatty liver disease (NAFLD) and associated fibroinflammation.
- BAT Activation and Reversal of Metabolic Comorbidities: Exploring molecular mechanisms related to brown adipocyte injury, BAT fibroinflammation, and its role in preventing or reversing metabolic complications like NASH.
The programme's uniqueness lies in several factors:
- Utilisation of insights from human genetics to identify genes associated with metabolically healthy obesity or unhealthy leanness, offering an integrated perspective on uncoupling obesity from complications.
- Pioneering research in lipotoxicity and phospholipid membrane composition in adipose tissue and the liver, bridging fields to propose a unified model for understanding obesity-related comorbidities.
- The application of cutting-edge high-throughput technologies and data analysis, coupled with expertise in murine metabolic phenotyping and in vitro models, enhances the efficiency of data generation and analysis.
- Strong collaborative network with national and international partners and industry, facilitating validation of findings in human cohorts and enhancing translatability.
Overall, the programme seeks to unravel the complex mechanisms underlying obesity-related metabolic complications, offering potential diagnostic biomarks and innovative therapeutic approaches for personalized obesity medicine.
Lay summary
Our research programme is dedicated to understanding the complex relationship between obesity and its impact on health. Beyond mere weight gain, obesity can lead to serious health issues such as diabetes and liver disease. We are focused on the underlying reasons why some individuals develop these complications while others do not. One of our key discoveries is that certain factors in our fat cells can trigger inflammation in various parts of the body, contributing to these health problems. By uncovering these mechanisms, we aim to develop novel ways to prevent and treat obesity-related complications. What distinguishes our work is our integration of cutting-edge technology with insights from genetics and mouse physiology and our collaboration with experts worldwide. Ultimately, our objective is to enhance personalised treatments for obesity-related health conditions, ensuring a healthier future for all.
Programme leaders: Professor Fiona Gribble and Professor Frank Reimann
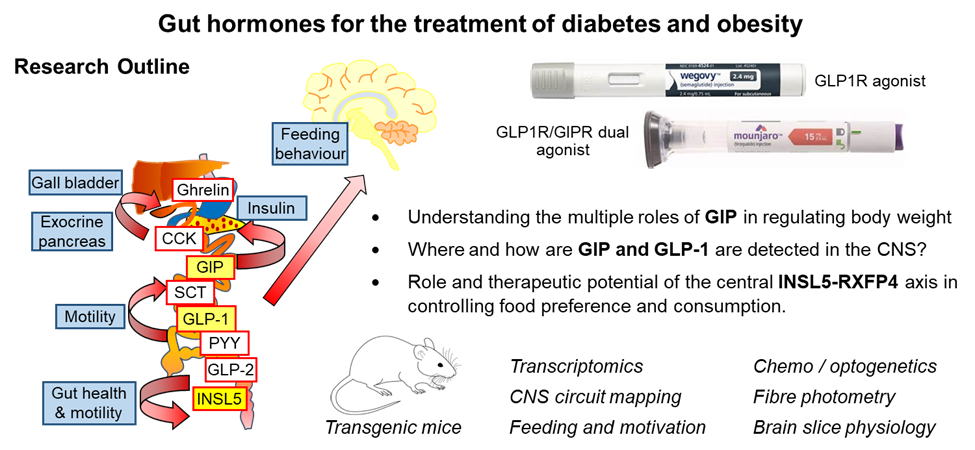
Long-acting incretin-based therapies have revolutionised treatment of type 2 diabetes and obesity. They harness hormones of the gut-brain-pancreatic axis to mimic the post-prandial state, including by stimulating glucose-dependent insulin secretion and reducing food intake. Most drugs in this class are based on the gut hormone glucagon-like peptide-1 (GLP-1), with newer drugs demonstrating improved efficacy by additionally targeting receptors for other metabolic hormones such as glucose-dependent insulinotropic polypeptide (GIP), glucagon and amylin.
Despite the clinical success of gut-hormone-based drugs, their mechanisms of action and central targets are incompletely understood. The aims of this programme are to identify central targets of gut hormones and incretin-based therapies, and the associated neural circuitry involved in control of food intake. Specifically, the programme will aim to identify pathways involved in the biology of three important gut-brain axes: GLP-1/GLP1R, GIP/GIPR, and INSL5/RXFP4. It will address questions such as: Where and how are GIP and GLP-1 are detected in the CNS? What are the physiological roles of endogenous GIP in the regulation of food intake? Why do both agonists and antagonists of GIPR result in body weight reduction? What is the physiological role and therapeutic potential of the central INSL5-RXFP4 axis (which has been shown previously to control food preference and consumption)?
The programme will utilise murine models in which specific neuronal populations in the brain can be modulated and monitored, enabling integrated assessments of behaviours such as feeding and motivation. Chemogenetics and optogenetics will be used to modulate specific neuronal populations in target central nuclei, using transgenic mouse strains developed in Cambridge (e.g. Glu-Cre/Glp1r-Cre, Gip-Cre/Gipr-Cre, Insl5-Cre/Rxfp4-Cre) which will be crossed with appropriate reporter strains or injected into target nuclei with AAVs to enable Cre-dependent expression of e.g. DREADDs or channel rhodopsins, as well as used for circuit mapping, single cell/nuclei RNA sequencing and calcium/cAMP imaging or electrophysiology of identified neuronal populations in brain slices. In other experiments we will use fibre photometry to monitor activity of specific neuronal populations in relation to feeding.
The programme has industrial partners focussed on understanding the physiology of incretin-based therapeutics, blood brain barrier penetration, and gut hormone measurement.
Lay summary
New treatments for obesity are based on mimicking the action of naturally occurring hormones from the gut, which send information about what and how much has been eaten, to the brain and pancreas. There are newer drugs in the pipeline which mimic the action of more than one gut hormone. Although clinical trials are demonstrating that these are even more effective at triggering weight loss, we do not understand the details about how they work. This programme aims to identify pathways in the brain which are activated by gut hormones, and to understand how they bring about weight loss and improvements in diabetes control. The studies will mostly be performed in live mice, in which feeding behaviour can be monitored after stimulating or blocking different points in the pathways controlling food intake. With improved understanding how the different gut hormones act on the brain, it is hoped that the science will promote the development of new drugs for diabetes and obesity that are more effective and have fewer side effects.
Programme leaders: Professor Sue Ozanne and Dr Miguel Constancia
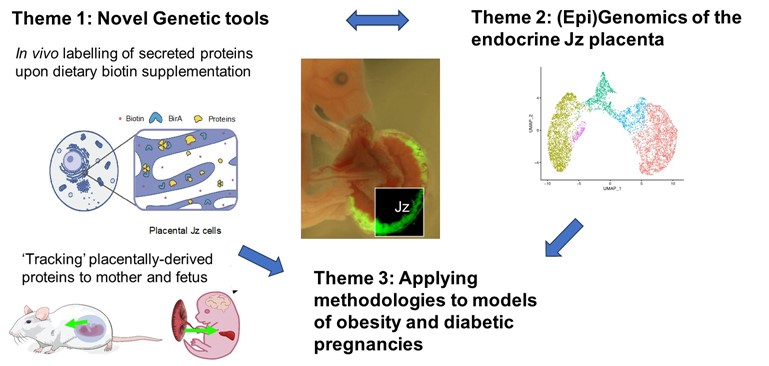
This programme builds on our recent work on the placenta as an endocrine organ that is a key determinant of maternal metabolism, an important organ that responds to nutritional cues during pregnancy and mediates effects on the fetus. How the placenta responds when exposed to a suboptimal environment can therefore influence the short and long-term health of the mother and offspring. Over half of pregnancies in the UK are affected by obesity/over-weight and globally one in seven babies are born from a gestational diabetic pregnancy. These conditions during pregnancy are associated with long-term poor cardiometabolic health in the mother and child. Understanding the molecular mechanisms by which the placenta integrates signals between mother and fetus is therefore important when considering strategies to improve maternal and fetal health. Our programme will focus on maternal-fetal communication via the placenta, the impact of obesity and hyperglycaemia on these processes and establish if any of these processes show sexual dimorphism. We have three specific aims (i) To identify and characterize secreted proteins from the feto-placental unit that traffic to maternal and fetal organs (ii) To discover key molecules and (epi)genetic mechanisms regulating placental endocrine function and (iii) To establish how maternal obesity alters inter-organ trafficking processes during pregnancy (including through alteration in protein and miRNA content of placental extracellular vesicles). We will achieve these aims by capitalising on newly developed transgenic mouse strains that allow us to interrogate protein trafficking from the placenta to maternal and fetal organs and state of the art genomic and epigenomic technologies (e.g. single cell nuclear RNA sequencing, single cell cut and tag technologies to profile histone modifications) combined with our established mouse model of obese, glucose intolerant pregnancy and our extensive experience in placental biology and murine cardiometabolic phenotyping. We will thus explore how dysregulated inter-organ communication during pregnancy modulates maternal and fetal metabolic health. This will provide mechanistic insight into potential strategies to improve the health of women and their children, recognising pregnancy as a window of opportunity to improve the health of at least two generations. These studies will contribute to the Unit’s mission to investigate mechanisms underlying metabolic disease and provide insight into the treatment and prevention of obesity and associated metabolic conditions.
Lay summary
Over half of women in the UK, and many settings globally, are overweight or living with obesity during pregnancy. This has immediate and long-term detrimental health consequences for both mother and baby, including increasing the risk of developing gestational diabetes and high blood pressure during pregnancy and increasing risk of heart disease and type 2 diabetes in later life. The placenta (that is made of both maternal and fetal cells) is an important link between mother and baby. It produces proteins and other molecules that are released into the maternal and fetal circulation to regulate their metabolism and maintain a healthy pregnancy. We think that during an obese pregnancy the proteins and other molecules produced by the placenta are altered and that these impact on the health of both mother and baby. Using mouse models and state-of-the-art technologies, we will measure the proteins released by the placenta during obese and healthy pregnancies and study how these affect the development of the fetus and alter the mum’s metabolism during pregnancy. Our results will help us understand why obesity during pregnancy influences maternal and offspring health and help us design treatments that will improve health of women and their children.
Programme leader: Dr Clemence Blouet
Dietary protein restriction increases energy intake across species and hunger in healthy humans. This might represent an adaptive response driven by the need to obtain protein when dietary supplies do not cover bodily needs. Since protein is diluted in the modern obesogenic environment, these mechanisms might contribute to the obesity epidemic. However, how the brain adapts to protein restriction to increase appetite remains largely unknown, and the contribution of these mechanisms to the control of food intake and energy homeostasis are unclear. Our aim is to characterise brain nutrient sensing pathways engage during protein restriction and involved in energy and glucose homeostasis to identify new therapeutic targets for the treatment of obesity and metabolic diseases. Our goal for the next 5 years is to characterise the sensing mechanisms and neural circuits engaged during mild protein restriction using a candidate-based approach informed by our ongoing studies and an unbiased discovery approach using activity-dependant neuronal labelling, sequencing, and circuit mapping, and determine the relevance of these circuits to human obesity and as targets for anti-obesity therapies.
Lay summary
We know the brain plays an important role in the regulation of appetite, and changes in the way the brain regulates appetite can affect weight gain and health. We are interested in understanding how the brain regulation of appetite is modulated by diet, in particular by proteins in the diet. Our goal is to identify the mechanisms through which the brain pathways regulating appetite are modulated by proteins using rodent models, determine if these pathways are important in human appetite control, and find ways to target these pathways to help treat obesity.